We are big advocates of making your own skincare, BUT we are also advocates of doing so safely. After all, most of us switch to making natural skincare products because we want a safer alternative to the potentially harmful chemicals in commercial products. What’s the point in switching if you’re putting yourself at risk by making products incorrectly?
One of the products we do NOT advise you to make or use is your own sunscreen.
We’re sorry if this is disappointing, but there are very good reasons not to.
The internet is full of DIY sunscreen recipes that won’t properly protect your skin. Home crafters even sell these products as sunscreens. Imagine the harm you could be doing to yourself by assuming these products are offering your skin adequate protection when they are not.
Take a few minutes now to become educated as to why DIY sunscreen is NOT a good idea.
This article will explain
How skin is damaged by UV rays
We’ve been reading and listening about the dangers of the sun for years now. Sunburn, accelerated skin aging and skin cancer are some of the possible consequences of excess exposure to the UV rays. Due to skin cancer rates being on the rise, the media mostly covers the negative aspects of sun exposure and neglects the positives one. Sun is essential for the formation of vitamin D (we cannot get sufficient vitamin D from food, and we require vitamin D for the maintenance of teeth and bones, for a healthy immune system, and for muscle function), it may benefit certain skin disorders (psoriasis, eczema) and it lifts our mood.
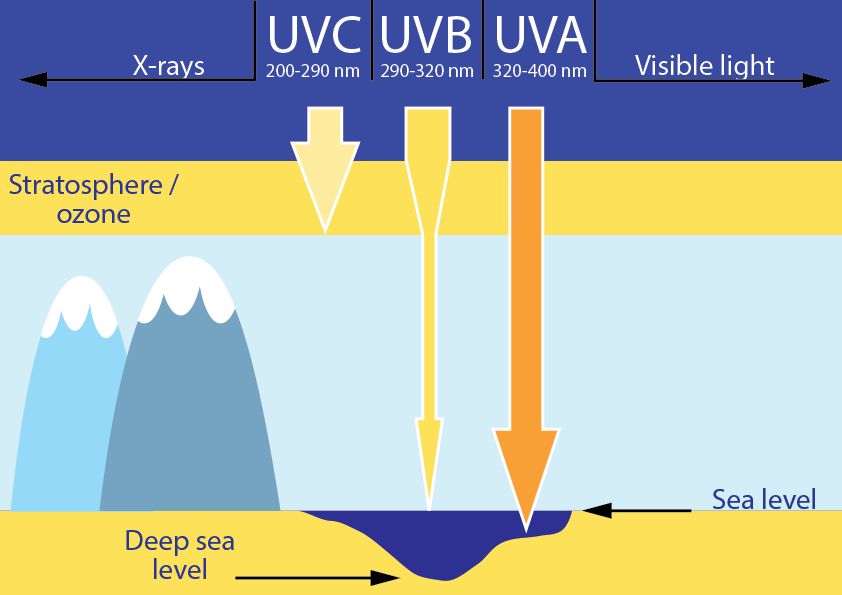
The source of all these effects of the sun is the UV light. We can divide UV rays into three subgroups – UVA, UVB and UVC.
UVC rays don’t reach the surface of the Earth as they are absorbed in the ozone layer.
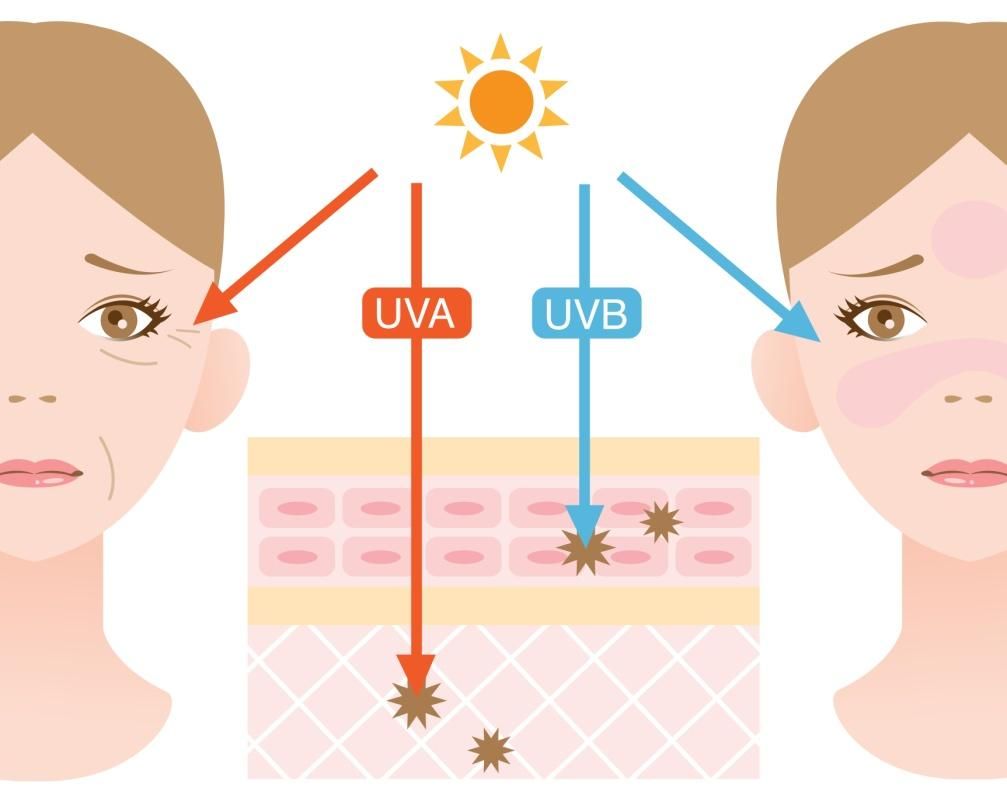
Some UVB rays are absorbed in the ozone layer, but a large fraction reaches the Earth. This spectrum range is responsible for sunburn and tanning of our skin, and also for skin aging and formation of certain types of skin cancer. UVB rays mainly affect the upper layers of the skin.
UVA rays easily penetrate the ozone layer and deep into our skin, where they cause aging of the skin, wrinkle formation and DNA damage (Runger, 2007). They also represent the majority of the UV light that reaches the Earth.
We usually think of sunscreen during the summer and when on seaside holidays, but it’s important to know that while the amount of UVB rays increase during the summer, the amount of UVA rays stay the same all year round!
How sunscreens work: UV filters
To protect us from unwanted effects of the sun on our skin, we use sunscreens – cosmetic products that contain UV filters. There are two types of UV filters: chemical and physical.
Most sunscreens contain so called chemical or organic UV filters; here are some examples you can find in the ingredient list of a sunscreen: octyl methoxycinnamate, oxybenzone, avobenzone, octisalate, octocrylene, homosalate, octinoxate.
The word ‘organic’ in the organic UV filters means that they are derived from organic chemistry (the opposite of inorganic chemistry), it has nothing to do with organic farming and organically sourced natural ingredients.
Chemical UV filters work by penetrating into the skin (this is why you need to apply sunscreen about 15 minutes before sun exposure) where they absorb the energy of the UV rays and protect form the sunburn. The mechanism of action is also the source of problems that surround chemical UV filters. They penetrate deep into the skin and possibly accumulate in soft tissues, they have been found in breast milk (Schlumpf et al., 2010) and in urine (Konusie et al., 2012). Several scientific experiments suggest that certain organic UV filters have endocrine disrupting properties (Krause et al., 2012) and may cause skin irritation (Bryden et al., 2006). Another issue concerning chemical UV filters is what happens after they absorb the energy of the UV light. The absorbed energy needs to be released somehow, and usually it’s through formation of free radicals, which cause DNA damage, premature aging and can even cause cancer (Hanson et al., 2006). To sum it up, chemical sunscreens protect from sunburns, but they do it at a price.
Especially with the popularity of natural skincare, inorganic or physical UV filters are becoming more and more important in sunscreens. They are also called mineral filters and they work by sitting on top of the skin and reflecting the UV rays away from it. The two mineral UV filters that are commonly used are zinc oxide and titanium dioxide. Because they do not penetrate the skin and they don’t absorb the UV light, they are much safer compared to chemical filters. Their main downside is the fact that they leave a white cast upon application on the skin.
To combat unwanted white cast left by mineral sunscreens researchers have developed nano sized zinc oxide and titanium dioxide. Nano UV filters have an average size below 100 nm and only reflect UV light, but not visible light, so they go onto the skin completely transparent. Nanotechnology is rather new and there are some concerns about it being used in skincare. One of the biggest issues people tend to refer to is the danger of nanoparticles penetrating the skin and accumulating in the body. Research (Cross et al., 2007) has shown that zinc oxide nanoparticles do not penetrate the skin, as long as it is healthy and intact. So from this perspective, using nano zinc oxide is not dangerous. On the other hand, it has been shown (Smijs & Pavel, 2011) that nanoparticles, especially titanium dioxide, form free radicals in the presence of light. This means that nano titanium dioxide can contribute to DNA damage and skin aging. To reduce this photoreactivity, most nanoparticles of zinc oxide and titanium dioxide are used in coated versions – the surface of the particles is coated with an inert compound, usually some type of silicone. This provides a lot of protection from free radical formation, but the technology and its use in sunscreens is still new so, more research will be needed to give more information on the safety of it.
Can you make DIY sunscreen with zinc oxide?
Zinc oxide is an easily accessible ingredient and is becoming very popular in DIY sunscreens. After all, store-bought mineral sunscreens contain zinc oxide, we can easily mix it into a lotion and create our own sunscreen, right?
Actually, no. Formulating and manufacturing a sunscreen is a lot more complicated than it might seem at first.
Firstly, safe and effective sunscreen formulation requires a very experienced cosmetic chemist.
Secondly, mineral filters have a tendency to clump together to form microscopic lumps. You won’t be able to see them with naked eye, but unless they are broken down properly, they are there. And what do lumps of zinc oxide mean for the sunscreen? When applied onto the skin, the skin won’t be completely and evenly covered, there will be gaps between the clumps where the sun can cause burns. This is why professional equipment is absolutely necessary – this means a high shear mixer/homogenizer; the price for this piece of equipment starts at $600. Regular kitchen stick blender simply won’t do the job. Also, special ingredients that work as dispersing agents, for example polyhydroxy stearic acid, are also vital for a mineral sunscreen.
Thirdly, any kind of sunscreen formula, mineral or chemical, needs to have its SPF tested in a lab. During the sunscreen formula development, you may get an in-vitro SPF prediction test, which costs $200-400. The final stage is a real SPF determination test. These tests are done on volunteers and cost from $2000 upwards. You might think that you don’t really need expensive tests if you are only making it for yourself and are not going to be selling your sunscreen. But the truth is that without a lab test, you can’t know what the SPF level is and you are playing Russian roulette with your skin.
The bottom line is, if you want to make sunscreen at home, you will need to have good knowledge of cosmetic chemistry and experience in formulating cosmetic products, proper equipment and the budget to have your product tested.
Do carrier oils have SPF levels?
Maybe you have read that certain plant oils and butters have high enough SPF and can be used as sunscreens. Unfortunately, this is an unproven myth. Most carrier oils have an SPF level about 1-3, which is far from enough for an effective sunscreen.
One of the reasons people think some carrier oils have good SPF properties is due to misinterpreting scientific studies, namely confusing in vitro absorbance measurements with in vivo SPF measurements.
In vitro absorbance measurements are *not* identical to in vivo SPF measurements. To explain a little bit: in vitro UV absorbance measures the amount of UV light certain material (in this case carrier oils) absorb. In vivo SPF measures the reaction of the skin to the UV light (redness or erythema) and at what dosage of UV light it appears on skin treated with sunscreen vs untreated skin. In vivo SPF measurements are the true test of how effective something is as a sunscreen.
With carrier oils it is difficult to just extrapolate absorbance measurements to SPF levels, because we do not know what happens when the oil is exposed to sunlight, air, and hot summer temperatures. In might start to oxidize and release free radicals that harm the skin. So, until actual in vivo measurement are done, there is no way of knowing how much protection from the sun carrier oils offer.
The most commonly quoted oils that are alleged to have high SPF levels include:
Raspberry seed oil
It is said it has an SPF of about 28-40. This is a result of misinterpretation of a scientific paper that only proved raspberry oil to have the same light absorbance as a SPF 28-40 sunscreen (Oomah et al., 2000), but this does not mean it will actually work as sunscreen when applied on the skin. As explained above, in vitro absorbance measurements are *not* identical to in vivo SPF measurements and you can’t just extrapolate absorbance measurements to SPF levels.
Carrot seed oil
There is one research paper (Kapoor et al., 2009) often cited as the source of information that carrot seed oil (not clear whether essential oil or fatty oil) has SPF of 38-40. But in reality, this experiment tested the SPF of a commercial sunscreen product that contained carrot seed oil among other ingredients. It is suspected that the actual ingredient responsible for this SPF was zinc oxide.
Coconut oil, olive oil, soybean oil
In various sources these oils are said to have SPF as high as 8, but it has been proven that in reality it is much lower, below 1 actually (Gause and Chauhan, 2016).
While vegetable oils, especially the ones that are high in antioxidants, can be a great addition to sunscreen formulation, they are not effective sunscreens on their own.
Three reasons not to make (or use) DIY sunscreen
There is much information on sunscreens and recipes for DIY versions online, but sadly, as we have explained, most of it is incorrect and even dangerous.
1) Carrier oils are not proven to have sufficient SPF levels. Articles quoting this are based on misinterpretation of scientific studies. Most carrier oils have an SPF level about 1-3, which is far from enough for an effective sunscreen.
2) Zinc oxide can’t just be mixed into a product to make a sunscreen. To work properly in a product, it must be dispersed very evenly, which is hard to achieve without professional homogenizers and the addition of dispersing agents, since it is inclined to form microscopic lumps, which then leave gaps of unprotected skin.
3) Sunscreen formulas need to have their SPF tested in a lab. You might think that you don’t really need expensive tests if you are only making it for yourself and are not going to be selling your sunscreen. But the truth is that without a lab test, you can’t know what the SPF level is and you are playing Russian roulette with your skin.
While making your own (safe and effective) sunscreen is not impossible, it is far from easy and requires an experienced formulator, expensive equipment, and even more expensive tests.
What sunscreen should you use?
Hopefully after reading this far you won’t try to make your own sunscreen unless you have the required expertise, equipment and tests and neither will you use a ‘homemade’ sunscreen sold by a home crafter/formulator that hasn’t undergone professional testing.
So what should you use instead?
There are risks connected to chemical UV filters as explained above, so an alternative is mineral sunscreen: look for products that only contain zinc oxide and/or titanium dioxide as UV filters on the label. Also, try to avoid the sun from 11am to 3pm or wear protective clothing during this time.
If you look for certified natural/organic sunscreen, it won’t contain chemical UV filters as those are not permitted in most well-known certifications. Plus, you will also be sure you are only applying naturally derived ingredients on your skin.
How to check the SPF of a sunscreen using official symbols
The level of protection will be listed as an SPF number on the product. This will tell you how effective the sunscreen is at protecting you from UVB rays. If the product protects from UVA rays as well, you will know by the wording “broad spectrum” (in the USA), by a symbol that has “UVA” written inside a circle (in the EU) or by UVA and stars in a circle symbol (in the UK).
In Japan and South Korea, the PA system is used to show the level of UVA protection – It ranges from PA+ to PA++++.
Some sunscreens also list how long they are water resistant for.
We hope you now feel more knowledgeable about which sunscreens to use and which to avoid, and can make an informed decision when choosing which one you’ll use.
References
Bryden AM, Moseley H, Ibbotson SH, Chowdhury MMU, et al. (2006). Photopatch testing of 1155 patients: results of the U.K. multicentre photopatch study group. British Journal of Dermatology, 155: 737-747.
Cross, S. E., Innes, B., Roberts, M. S., Tsuzuki, T., Robertson, T. A., & McCormick, P. (2007). Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacology and Physiology, 20(3): 148-154.
Gause, S., & Chauhan, A. (2016). UV-blocking potential of oils and juices. International Journal of Cosmetic Science, 38(4): 354-363.
Hanson KM, Gratton E, Bardeen CJ. (2006). Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radical Biology & Medicine, 41(8): 1205-1212.
Kapoor, S., & Saraf, S. (2009). Efficacy study of sunscreens containing various herbs for protecting skin from UVA and UVB sun rays. Pharmacognosy Magazine, 5(19): 238.
Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. (2012). Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. International Journal of Andrology, 35: 424-436.
Kunisue T, Chen Z, Buck Louis G, Sundaram R, Hediger M, Sun L, Kannan K. (2012). Urinary Concentrations of Benzophenone-type UV Filters in US Women and Their Association with Endometriosis. Environmental Science and Technology, 46(8): 4624-32. DOI: 10.1021/es204415a
Oomah, B. D., Ladet, S., Godfrey, D. V., Liang, J., & Girard, B. (2000). Characteristics of raspberry (Rubus idaeus L.) seed oil. Food chemistry, 69(2): 187-193.
Rünger, T. M. (2007). How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. Journal of Investigative Dermatology, 127(9): 2103-2105.
Schlumpf, M., Kypke, K., Wittassek, M., Angerer, J., Mascher, H., Mascher, D., Lichtensteiger, W. (2010). Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere, 81(10): 1171-1183.
Smijs, T. G., & Pavel, S. (2011). Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnology, Science and Applications, 4: 95-122.